The energy needed to remove one or more electrons from a neutral atom to form a positively charged ion is a physical property that influences the chemical behavior of the atom. By definition, the first ionization energy of an element is the energy needed to remove the outermost, or highest energy, electron from a neutral atom in the gas phase.
The process by which the first ionization energy of hydrogen is measured would be represented by the following equation.
| H(g) |
| Practice Problem 3:Use the Bohr model to calculate the wavelength and energy of the photon that would have to be absorbed to ionize a neutral hydrogen atom in the gas phase. |
| CH4(g) + 2 O2(g) |
| Fe2O3(s) + 2 Al(s) |
The first ionization energy for helium is slightly less than twice the ionization energy for hydrogen because each electron in helium feels the attractive force of two protons, instead of one.
| He(g) |
| Li(g) |
The first ionization energies for the main group elements are given in the two figures below.
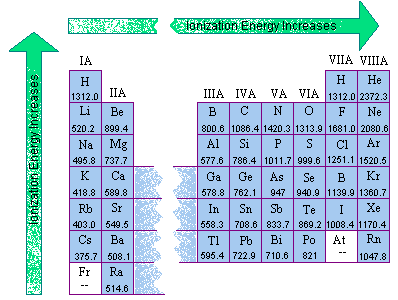 | 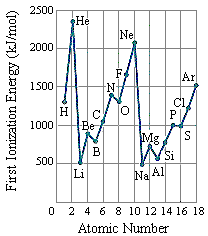 |
- In general, the first ionization energy increases as we go from left to right across a row of the periodic table.
- The first ionization energy decreases as we go down a column of the periodic table.
The second trend results from the fact that the principal quantum number of the orbital holding the outermost electron becomes larger as we go down a column of the periodic table. Although the number of protons in the nucleus also becomes larger, the electrons in smaller shells and subshells tend to screen the outermost electron from some of the force of attraction of the nucleus. Furthermore, the electron being removed when the first ionization energy is measured spends less of its time near the nucleus of the atom, and it therefore takes less energy to remove this electron from the atom.
The figure below shows the first ionization energies for elements in the second row of the periodic table. Although there is a general trend toward an increase in the first ionization energy as we go from left to right across this row, there are two minor inversions in this pattern. The first ionization energy of boron is smaller than beryllium, and the first ionization energy of oxygen is smaller than nitrogen.
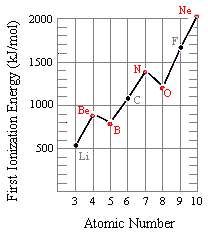
Be: [He] 2s2
B: [He] 2s2 2p1
The electrons removed when nitrogen and oxygen are ionized also come from 2p orbitals. N: [He] 2s2 2p3
O: [He] 2s2 2p4
But there is an important difference in the way electrons are distributed in these atoms. Hund's rules predict that the three electrons in the 2p orbitals of a nitrogen atom all have the same spin, but electrons are paired in one of the 2p orbitals on an oxygen atom. 
| Practice Problem 4:Predict which element in each of the following pairs has the larger first ionization energy. (a) Na or Mg (b) Mg or Al (b) Mg or Al (c) F or Cl |
By now you know that sodium forms Na+ ions, magnesium forms Mg2+ ions, and aluminum forms Al3+ ions. But have you ever wondered why sodium doesn't form Na2+ ions, or even Na3+ ions? The answer can be obtained from data for the second, third, and higher ionization energies of the element.
The first ionization energy of sodium, for example, is the energy it takes to remove one electron from a neutral atom.
Na(g) + energy  Na+(g) + e-
Na+(g) + e-
The second ionization energy is the energy it takes to remove another electron to form an Na2+ ion in the gas phase. Na+(g) + energy  Na2+(g) + e-
Na2+(g) + e-
The third ionization energy can be represented by the following equation. Na2+(g) + energy  Na3+(g) + e-
Na3+(g) + e-
The energy required to form a Na3+ ion in the gas phase is the sum of the first, second, and third ionization energies of the element. First, Second, Third, and Fourth Ionization Energies
of Sodium, Magnesium, and Aluminum (kJ/mol)
of Sodium, Magnesium, and Aluminum (kJ/mol)

A similar pattern is observed when the ionization energies of magnesium are analyzed. The first ionization energy of magnesium is larger than sodium because magnesium has one more proton in its nucleus to hold on to the electrons in the 3s orbital.
Mg: [Ne] 3s2
The second ionization energy of Mg is larger than the first because it always takes more energy to remove an electron from a positively charged ion than from a neutral atom. The third ionization energy of magnesium is enormous, however, because the Mg2+ ion has a filled-shell electron configuration. The same pattern can be seen in the ionization energies of aluminum. The first ionization energy of aluminum is smaller than magnesium. The second ionization energy of aluminum is larger than the first, and the third ionization energy is even larger. Although it takes a considerable amount of energy to remove three electrons from an aluminum atom to form an Al3+ ion, the energy needed to break into the filled-shell configuration of the Al3+ ion is astronomical. Thus, it would be a mistake to look for an Al4+ ion as the product of a chemical reaction.
| Practice Problem 5:Predict the group in the periodic table in which an element with the following ionization energies would most likely be found. 1st IE = 786 kJ/mol 2nd IE = 1577 3rd IE = 3232 4th IE = 4355 5th IE = 16,091 6th IE = 19,784 |
Practice Problem 6:Use the trends in the ionization energies of the elements to explain the following observations.
(a) Elements on the left side of the periodic table are more likely than those on the right to form positive ions.
(b) The maximum positive charge on an ion is equal to the group number of the element