Ionization energies measure the tendency of a neutral atom to resist the loss of electrons. It takes a considerable amount of energy, for example, to remove an electron from a neutral fluorine atom to form a
positively charged ion.
F(g)  F+(g) + e- F+(g) + e- |
|
|
|  Ho = 1681.0 kJ/mol Ho = 1681.0 kJ/mol |
The
electron affinity of an element is the energy given off when a neutral atom in the gas phase gains an extra electron to form a
negatively charged ion. A fluorine atom in the gas phase, for example, gives off energy when it gains an electron to form a fluoride ion.
F(g) + e-  F-(g) F-(g) |
|
|
|  Ho = -328.0 kJ/mol Ho = -328.0 kJ/mol |
Electron affinities are more difficult to measure than ionization energies and are usually known to fewer significant figures. The electron affinities of the main group elements are shown in the figure below.
Several patterns can be found in these data.
- Electron affinities generally become smaller as we go down a column of the periodic table for two reasons. First, the electron being added to the atom is placed in larger orbitals, where it spends less time near the nucleus of the atom. Second, the number of electrons on an atom increases as we go down a column, so the force of repulsion between the electron being added and the electrons already present on a neutral atom becomes larger.
- Electron affinity data are complicated by the fact that the repulsion between the electron being added to the atom and the electrons already present on the atom depends on the volume of the atom. Among the nonmetals in Groups VIA and VIIA, this force of repulsion is largest for the very smallest atoms in these columns: oxygen and fluorine. As a result, these elements have a smaller electron affinity than the elements below them in these columns as shown in the figure below. From that point on, however, the electron affinities decrease as we continue down these columns.

At first glance, there appears to be no pattern in electron affinity across a row of the periodic table, as shown in the figure below.
When these data are listed along with the electron configurations of these elements, however, they make sense. These data can be explained by noting that electron affinities are much smaller than ionization energies. As a result, elements such as helium, beryllium, nitrogen, and neon, which have unusually stable electron configurations, have such small affinities for extra electrons that no energy is given off when a neutral atom of these elements picks up an electron. These configurations are so stable that it actually takes energy to force one of these elements to pick up an extra electron to form a negative ion.
Electron Affinities and Electron Configurations for the First 10 Elements in the Periodic Table
| Element |
| Electron Affinity (kJ/mol) |
| Electron Configuration |
|
|
|
| H |
| 72.8 |
| 1s1 |
|
|
|
| He |
| <0 td=""> |
| 1s2 |
|
|
|
| Li |
| 59.8 |
| [He] 2s1 |
|
|
|
| Be |
| <0 td=""> |
| [He] 2s2 |
|
|
|
| B |
| 27 |
| [He] 2s2 2p1 |
|
|
|
| C |
| 122.3 |
| [He] 2s2 2p2 |
|
|
|
| N |
| <0 td=""> |
| [He] 2s2 2p3 |
|
|
|
| O |
| 141.1 |
| [He] 2s2 2p4 |
|
|
|
| F |
| 328.0 |
| [He] 2s2 2p5 |
|
|
|
| Ne |
| <0 td=""> |
| [He] 2s2 2p6 |
|
|
|
|
|
|
|
|
|
|
|
Students often believe that sodium reacts with chlorine to form Na
+ and Cl
- ions because chlorine atoms "like" electrons more than sodium atoms do. There is no doubt that sodium reacts vigorously with chlorine to form NaCl.
2 Na(
s) + Cl
2(
g)

2 NaCl(
s)
Furthermore, the ease with which solutions of NaCl in water conduct electricity is evidence for the fact that the product of this reaction is a salt, which contains Na
+ and Cl
- ions.
| NaCl(s) | H2O | Na+(aq) + Cl-(aq) |
|
The only question is whether it is legitimate to assume that this reaction occurs because chlorine atoms "like" electrons more than sodium atoms.
The first ionization energy for sodium is one and one-half times larger than the electron affinity for chlorine.
Na: 1st IE = 495.8 kJ/mol
Cl: EA = 328.8 kJ/mol
Thus, it takes more energy to remove an electron from a neutral sodium atom than is given off when the electron is picked up by a neutral chlorine atom. We will obviously have to find another explanation for why sodium reacts with chlorine to form NaCl. Before we can do this, however, we need to know more about the chemistry of ionic compounds.
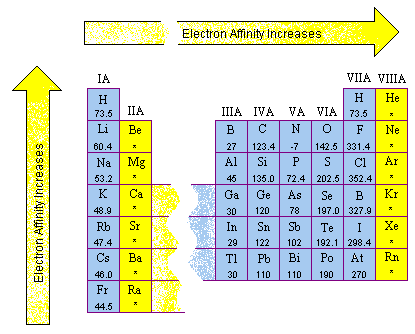 Several patterns can be found in these data.
Several patterns can be found in these data.  At first glance, there appears to be no pattern in electron affinity across a row of the periodic table, as shown in the figure below.
At first glance, there appears to be no pattern in electron affinity across a row of the periodic table, as shown in the figure below. 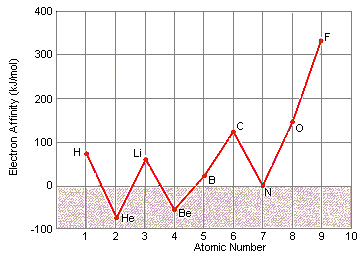 When these data are listed along with the electron configurations of these elements, however, they make sense. These data can be explained by noting that electron affinities are much smaller than ionization energies. As a result, elements such as helium, beryllium, nitrogen, and neon, which have unusually stable electron configurations, have such small affinities for extra electrons that no energy is given off when a neutral atom of these elements picks up an electron. These configurations are so stable that it actually takes energy to force one of these elements to pick up an extra electron to form a negative ion.
When these data are listed along with the electron configurations of these elements, however, they make sense. These data can be explained by noting that electron affinities are much smaller than ionization energies. As a result, elements such as helium, beryllium, nitrogen, and neon, which have unusually stable electron configurations, have such small affinities for extra electrons that no energy is given off when a neutral atom of these elements picks up an electron. These configurations are so stable that it actually takes energy to force one of these elements to pick up an extra electron to form a negative ion. 


 2 NaCl(s)
2 NaCl(s)