Electronic Configuration of Elements
Mendeleev noticed the recurrence of properties of elements as the atomic weight increased, and he invented the Periodic Table of Element, which is a useful tool for organizing and correlating chemical and physical properties of chemical elements. Today, the most popular Periodic Table form is shaped by results of quantum theory. Quantum theory rationalized the existence of and arrangement of all elements in today's Periodic Table. It has also been applied to explain their chemical properties.
Electronic Configuration of Elements
Skills to develop
- Explain the rules for filling electrons in atomic orbitals -- Pauli exclusion principle and Hund's rule
- Fill electrons in atomic orbitals--Aufbau process
- Explain the arrangement of elements in terms of quantum numbers
- Explain the systematic variation of element properties
Energy Levels in Many-Electron Atoms
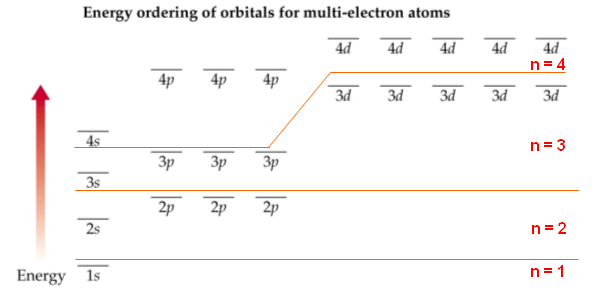
In order to fill the electrons in various atomic orbitals, we need to know how the energy levels vary as the nuclear charge increases. For hydrogen-like atoms, the approximate energy levels are as indicated below:
The shielding effect and electron-electron interactions cause the energy levels of subshells such as 2s & 2p to be different from those of H-like atoms. This is done by treating the electron shield cores as a proton but the core has an effective nuclear charge Z.
 For the H-like atoms, energy levels for 2s, 2p stay the same, but the separation between 2s and 2p energy levels increases as the atomic number (Z) increases. Similar situations happen for 3s, 3p, and 3d energy levels. The energy diagrams of H, Li & K are used to illustrate this point. The color diagram is from a Hyperion website discussing quantum numbers and structure of atoms
For the H-like atoms, energy levels for 2s, 2p stay the same, but the separation between 2s and 2p energy levels increases as the atomic number (Z) increases. Similar situations happen for 3s, 3p, and 3d energy levels. The energy diagrams of H, Li & K are used to illustrate this point. The color diagram is from a Hyperion website discussing quantum numbers and structure of atoms Understand how the energy level vary is the key to the Aufbau process, because Electrons tend to occupy the lowest energy level available. But before we talk about the Aufbau process, we need to be aware of the Pauli exclusion principle and the Hund's rule.
The Pauli Exclusion Principle
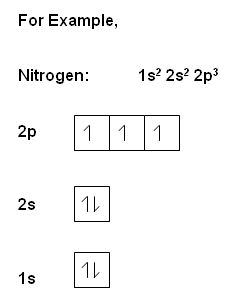
The Pauli exclusion principle suggests that only two electrons with opposite spin can occupy an atomic orbital. Stated another way, no two electrons have the same 4 quantum numbers n, l, m, s. Pauli's exclusion principle can be stated in some other ways, but the idea is that energy states have limit room to accommodate electrons. A state accepts two electrons of different spins.In applying this rule, You should realize that an atomic orbital is an energy state.
Hund's RULE
Hund's rule suggests that electrons prefer parallel spins in separate orbitals of subshells. This rule guides us in assigning electrons to different states in each sub-shell of the atmic orbitals. In other words, electrons fill each and all orbitals in the subshell before they pair up with opposite spins. Pauli exclusion principle and Hund's rule guide us in the aufbau process, which is figuring out the electron configurations for all elements.The Aufbau Procedure
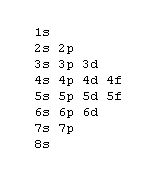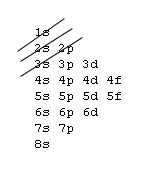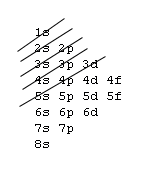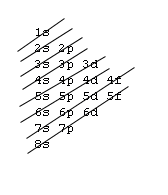
The aufbau procedure (filling order of atomic orbitals) is used to work out the electron confiturations of all atoms. However, modification should be made by applying Hund's rule to be discussed in the next section. The aufbau procedure is based on a rough energy levels diagram of many-electron atoms as shown below:7s |~ 5d |----- 5f |_______ Actanides 6s |_ 6p |~~~ 5d |===== 4f |======= Lanthanides 5s |_ 5p |~~~ 4d |----- Note the trend 5s 4d 5p & develope a pattern 4s |_ 4p |~~~ 3d |----- Transition elements 3s |_ 3p |--- 2s |_ 2p |--- 1s |-The fine difference between energy levels cannot be adquately shown.
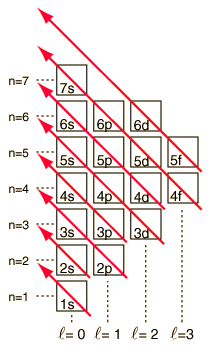
You've learned various techniques to work out the electronic configurations of elements. Here is yet another form of an energy level diagram:
7p _ _ _
7s _ 5f - - - - - - - 6d ~ ~ ~ ~ ~
6p _ _ _
6s _ 4f - - - - - - - 5d ~ ~ ~ ~ ~
5s _ 4d - - - - - 5p ~ ~ ~
4s _ 3d - - - - - 4p ~ ~ ~
3s _ 3p - - -
2s _ 2p - - -
1s _
In order to master the technique of the aufbau procedure, you should apply the Hund's rule and Pauli exclusion principle to work out the electronic configuration for closed shells of inert gases. After you worked out, you may compare your result with the following. Do not try to remember it, it is important to know how to work it out. Z= 2 10 18 36 54 86
1s2 2s22p6 3s23p6 4s23d104p6 5s24d105p6 6s24f145d106p6
He Ne Ar Kr Xe Rn
Block of elements by highest occupied atomic orbitals
| Block of elements by last filled atomic orbitals | |||
|---|---|---|---|
| 1s 2s 3s 4s 5s 6s 7s | 4f - - - - - 4f 5f - - - - - 5f | 3d - - - 3d 4d - - - 4d 5d - - - 5d 6d - - - 6d | 2p - 2p 3p - 3p 4p - 4p 5p - 5p 6p - 6p 7p - 7p |
In an ordinary periodic table, the s, p, and d block elements are in the main body of the Periodic Table, whereas the f block elements are placed below the main body. If we placed them on the same period where they belong, the Periodic Table would be too long for the screen to accommodate. Thus, we keep the Periodic Table in the usual (long) form.
Special Electronic Configurations
When two electrons occupy the same orbital, they not only have different spins (Pauli exclusion principle), the pairing raises the energy slightly. On the other hand, a half filled subshell and a full filled subshell lower the energy, gaining some stability. Bearing this in mind, you will be able to understand why we have the following special electronic configurations.| Cr [Ar]4s1 3d5 | <=All s and d subshells are half full |
| Cu [Ar]4s1 3d10 | <=Prefers a filled d subshell, leaving s with 1 |
| Nb [Kr]5s1 4d4 | <=5s and 4d energy levels are close |
| Mo [Kr]5s1 4d5 | similar to Cr above |
| Tc [Kr]5s2 4d5 | (not special, but think of Hund's rule) |
| Ru [Kr]5s1 4d7 | <= Only 1 5s electron |
| Rh [Kr]5s1 4d8 | <= in both |
| Pd [Kr]5s0 4d10 | <= Note filled 4d and empty 5s |
| Ag [Kr]5s1 4d10 | <= partial filled 5s, but filled d |
Confidence Building Questions
- For the H-like atom, which subshell has the highest energy level?
4f, 3d, 2p, 1s Skill:
Describe the energy levels of hydrogen atoms. - For an element with atomic number 20, which is the last or highest occupied subshell of atomic orbitals?
1s 2s 2p 3s 3p 3d 4s 5s Discussion:
The element is Calcium, Ca. - How many electrons are required to fill all the following sub shells?
1s 2s 2p 3s 3p 4s 3d 4p Discussion:
The element is Krypton, Kr. - Which of the following two electronic configuration is more stable?
a [Ar]4s1 3d5
b [Ar]4s2 3d4 Discussion:
Check the electronic configuration for Cu and Cr. - Which of the following two electronic configurations is more stable?
a [Ar]4s2 3d9
b [Ar]4s1 3d10 Discussion:
This is the electronic configuration for copper. - Choose the electronic configuration for palladium, Pd (Z = 46).
a [Kr]5s1 4d7
b [Kr]5s1 4d8
c [Kr]5s0 4d10
d [Kr]5s1 4d10
 ) have the lowest energy and will fill up first. Within a shell, there may be several orbitals with the same principal quantum number. In that case, more specific rules must be applied. For example, the three p orbitals of a given shell all occur at the same energy level. So, how are they filled up? ans: all the three p orbitals have same energy so while filling the p orbitals we can fill any one of the Px, Py or Pz first. it is a convention that we chose to fill Px first ,then Py and then Pz for our simplicity. Hence you can opt for filling these three orbitals from right to left also.
) have the lowest energy and will fill up first. Within a shell, there may be several orbitals with the same principal quantum number. In that case, more specific rules must be applied. For example, the three p orbitals of a given shell all occur at the same energy level. So, how are they filled up? ans: all the three p orbitals have same energy so while filling the p orbitals we can fill any one of the Px, Py or Pz first. it is a convention that we chose to fill Px first ,then Py and then Pz for our simplicity. Hence you can opt for filling these three orbitals from right to left also.










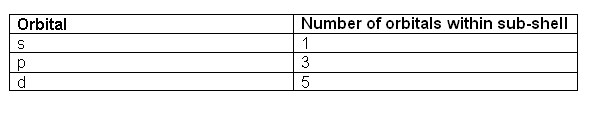
 , the electrons within the same orbital spin in opposite directions.
, the electrons within the same orbital spin in opposite directions.